Androgenetic alopecia

What causes AGA?
Clinical Phenomenology
Androgenetic alopecia in syndromes
Premature baldness
AHA classifications
What are the hallmark characteristics of AGA?
What is the normal hair growth cycle?
Follicle Health & Hair Growth
Hair follicle miniaturization
Relationship with other states
What Causes Postpartum Hair Loss?
What Causes Hair Loss in Menopause?
Can Stress Cause Hair to Fall Out?
What don’t we know about AGA?
What causes DHT to increase in balding scalp regions?
Where is AGA research heading?
Diagnosis
What are the treatment targets for AGA?
Аdjuvant therapy
Conclusions
Clinical Phenomenology
Androgenetic alopecia in syndromes
Premature baldness
AHA classifications
What are the hallmark characteristics of AGA?
What is the normal hair growth cycle?
Follicle Health & Hair Growth
Hair follicle miniaturization
Relationship with other states
What Causes Postpartum Hair Loss?
What Causes Hair Loss in Menopause?
Can Stress Cause Hair to Fall Out?
What don’t we know about AGA?
What causes DHT to increase in balding scalp regions?
Where is AGA research heading?
Diagnosis
What are the treatment targets for AGA?
Аdjuvant therapy
Conclusions
Androgenetic alopecia (AGA) is a complex polygenic multifactorial condition that is the most common form of hair loss, affecting up to 80% of men and 50% of women during their lifetime. AGA develops over the years and is a reflection of segmental or organ-specific premature aging.
What causes AGA?
While the exact step-processes of AGA aren’t yet determined, there is general consensus on two contributing factors: genetics and androgens (i.e., male hormones).
Genetic testing
AGA has an undisputed genetic component, with one study measuring a 2.5-fold increased risk of developing pattern hair loss in men whose fathers had pattern hair loss, as compared to those whose fathers didn’t (Chumlea et al., 2004). Moreover, women affected by AGA often report having other family members affected by the same condition (Ramos et al., 2015).
Genetic studies of this disease have brought to the fore the role of inheritance. In this regard, a recent analysis of published AGA genetic studies revealed a new association of AGA and rs7349332 located in the intron region of WNT10A, suggesting the involvement of WNT signaling in the etiology of AGA. In a German case-control study of AGA, it was shown that one of the reasons for the genetic risk of developing AGA is a pronounced polygenic component. This fact likely reflects the complexity of the biological pathways associated with AGA.
However, the exact genes involved in androgenic alopecia have not yet been discovered. The "commonly suspected" gene on the "androgen side" in this case is the androgen receptor (AR) gene, which is located on the X chromosome, which may explain maternal transmission of AGA. It is still unclear which genes other than AR are responsible for AGA. Patients should be aware that current genetic testing relies on variations in the AR gene, while the actual onset of androgenetic alopecia is caused by polygenic involvement of other genes or epigenetic mechanisms.
Scientists are still trying to uncover which polymorphisms may prompt individual susceptibility to AGA. Particular focus is on genes that code for androgen receptors (more on this soon).
Genetic studies of this disease have brought to the fore the role of inheritance. In this regard, a recent analysis of published AGA genetic studies revealed a new association of AGA and rs7349332 located in the intron region of WNT10A, suggesting the involvement of WNT signaling in the etiology of AGA. In a German case-control study of AGA, it was shown that one of the reasons for the genetic risk of developing AGA is a pronounced polygenic component. This fact likely reflects the complexity of the biological pathways associated with AGA.
However, the exact genes involved in androgenic alopecia have not yet been discovered. The "commonly suspected" gene on the "androgen side" in this case is the androgen receptor (AR) gene, which is located on the X chromosome, which may explain maternal transmission of AGA. It is still unclear which genes other than AR are responsible for AGA. Patients should be aware that current genetic testing relies on variations in the AR gene, while the actual onset of androgenetic alopecia is caused by polygenic involvement of other genes or epigenetic mechanisms.
Scientists are still trying to uncover which polymorphisms may prompt individual susceptibility to AGA. Particular focus is on genes that code for androgen receptors (more on this soon).
Androgen activity
Male hormones (i.e., testosterone) are closely tied to AGA. In the 1940’s, researchers observed that (Hamilton et al., 1942):
Thirty years later, scientists uncovered the specific male hormone involved in AGA: dihydrotestosterone (DHT).
- Men castrated before puberty (i.e., before their sex hormones spike) never develop AGA later in life.
- Men with AGA who are castrated (and thereby lose 95% of androgen production) also see a stop in AGA progression.
- Castrated men who are injected with testosterone begin to develop temple recession and/or vertex thinning.
Thirty years later, scientists uncovered the specific male hormone involved in AGA: dihydrotestosterone (DHT).
Dihydrotestosterone (DHT)
DHT – a metabolite of testosterone – is causally linked to pattern hair loss (English, 2018).
- Men who cannot make scalp DHT never develop AGA.
- Balding scalp regions have higher amounts of DHT versus non-balding regions.
- Drugs that reduce scalp DHT improve AGA outcomes in 80-90% of men.
However, not all types of DHT are implicated in pattern hair loss. Rather, research is focused more so on one specific type of DHT: DHT made from an enzyme called type II 5-alpha reductase.
Type II 5-alpha reductase
In the body, nearly all DHT is created when unbound testosterone comes into contact with the enzyme 5-alpha reductase. This enzyme binds to free testosterone and then changes testosterone’s structure into DHT.
There are different types of 5-alpha reductase, and most types correspond to a specific tissue or region in which the enzyme expresses (i.e., the skin, brain, prostate, etc.).
When it comes to AGA, the type II 5-alpha reductase is most heavily implicated.
This is because:
(1) type II 5-alpha reductase is greatly expressed in scalp tissues, and
(2) men with a gene mutation who cannot produce type II 5-alpha reductase never go bald (Adachi et al., 1970).
Moreover, drugs that inhibit the type II 5-alpha reductase enzyme (i.e., finasteride) help to improve pattern hair loss in men.
Together, these findings implicate DHT and the type II 5-alpha reductase enzyme in AGA. But there’s at least one more androgenic factor involved in the onset and progression of pattern hair loss: that of androgen receptors.
There are different types of 5-alpha reductase, and most types correspond to a specific tissue or region in which the enzyme expresses (i.e., the skin, brain, prostate, etc.).
When it comes to AGA, the type II 5-alpha reductase is most heavily implicated.
This is because:
(1) type II 5-alpha reductase is greatly expressed in scalp tissues, and
(2) men with a gene mutation who cannot produce type II 5-alpha reductase never go bald (Adachi et al., 1970).
Moreover, drugs that inhibit the type II 5-alpha reductase enzyme (i.e., finasteride) help to improve pattern hair loss in men.
Together, these findings implicate DHT and the type II 5-alpha reductase enzyme in AGA. But there’s at least one more androgenic factor involved in the onset and progression of pattern hair loss: that of androgen receptors.

Androgen receptors
When free testosterone comes into contact with type II 5-alpha reductase and converts into DHT, that DHT needs a place to bind to a cell. Once DHT is bound to a cell, this hormone can begin to influence that cell’s functionality.
Androgen receptors are where DHT binds to cells. They’re considered cellular “landing pads” – a place for male hormones to attach, so that they can begin to change cellular behavior.
That means that for (most) scalp DHT to form, we need:
(1) free testosterone,
(2) type II 5-alpha reductase, and
(3) an androgen receptor.
Androgen receptors are where DHT binds to cells. They’re considered cellular “landing pads” – a place for male hormones to attach, so that they can begin to change cellular behavior.
That means that for (most) scalp DHT to form, we need:
(1) free testosterone,
(2) type II 5-alpha reductase, and
(3) an androgen receptor.

This is why androgen receptors are a major focus of AGA research: without androgen receptors, DHT cannot bind to scalp cells and influence their functionality.
“DHT sensitivity”
Type II 5-alpha-DHT has a 5x higher affinity for the androgen receptor than other hormones like testosterone (Trüeb, 2002). This means that in balding scalps, DHT will begin preferentially bind to androgen receptors and exert more effects on cell function, at least compared to other male hormones.
This is why some researchers speak of AGA sufferers developing a “genetic sensitivity” to DHT. In the scalp, the more type II 5-alpha-DHT bound to androgen receptors, the more likely a person is to suffer from pattern hair loss.
This is why some researchers speak of AGA sufferers developing a “genetic sensitivity” to DHT. In the scalp, the more type II 5-alpha-DHT bound to androgen receptors, the more likely a person is to suffer from pattern hair loss.
Is DHT also involved in female pattern hair loss?
DHT is still involved, but there’s debate over its degree of involvement.
Some studies have demonstrated that both men and women have elevated levels of 5α-reductase in the frontal hair follicles (Orme et al, 1999, Sawaya et al., 1997). This suggests that DHT may play a role in at least some female AGA cases.
At the same time, case studies have demonstrated female pattern hair loss can occur in women who are androgen-deprived, meaning they lack the ability to produce any male hormones at all (Orme et al., 1999).
While some have argued that these instances are just cases of mistaken identity (i.e., not AGA), others have used these findings as starting points to explore other aspects of AGA – and what the DHT-genetic sensitivity argument might not be telling us.
Some studies have demonstrated that both men and women have elevated levels of 5α-reductase in the frontal hair follicles (Orme et al, 1999, Sawaya et al., 1997). This suggests that DHT may play a role in at least some female AGA cases.
At the same time, case studies have demonstrated female pattern hair loss can occur in women who are androgen-deprived, meaning they lack the ability to produce any male hormones at all (Orme et al., 1999).
While some have argued that these instances are just cases of mistaken identity (i.e., not AGA), others have used these findings as starting points to explore other aspects of AGA – and what the DHT-genetic sensitivity argument might not be telling us.
Other potential factors: prostaglandin D2, retinoid receptors, and PPAR pathways
A new wave of AGA research is focusing more so on non-androgenic factors that might influence hair follicle functionality.
In 2014, excitement was generated over prostaglandin D2 and its potential connection to pattern hair loss. Prostaglandin D2 is a fatty acid derivative that, theoretically, can express as a result of androgenic activity (i.e., DHT) (Nieves et al., 2014).
Prostaglandin D2 was once found to be elevated in balding scalp regions and even impede hair lengthening. However, follow-up studies have shown conflicting findings – suggesting that prostaglandin D2 might be less involved in AGA than initially believed (Villarreal-Villarreal et al., 2019).
Interestingly, evidence is accumulating that in addition androgenic activity, retinoid receptors and the PPAR pathway are intimately tied to hair follicle miniaturization – and may even explain why some women develop AGA despite having normal scalp DHT levels (Siu-Yin Ho et al., 2019).
In 2014, excitement was generated over prostaglandin D2 and its potential connection to pattern hair loss. Prostaglandin D2 is a fatty acid derivative that, theoretically, can express as a result of androgenic activity (i.e., DHT) (Nieves et al., 2014).
Prostaglandin D2 was once found to be elevated in balding scalp regions and even impede hair lengthening. However, follow-up studies have shown conflicting findings – suggesting that prostaglandin D2 might be less involved in AGA than initially believed (Villarreal-Villarreal et al., 2019).
Interestingly, evidence is accumulating that in addition androgenic activity, retinoid receptors and the PPAR pathway are intimately tied to hair follicle miniaturization – and may even explain why some women develop AGA despite having normal scalp DHT levels (Siu-Yin Ho et al., 2019).
Evidence implicates genes and androgen activity in the onset of AGA.
The hormone DHT is causally linked to AGA. Men who can’t produce DHT never go bald, DHT levels are higher in the scalps of balding men, and drugs that reduce scalp DHT help to stop AGA’s progression.
Scalp DHT is formed when free testosterone comes into contact with the enzyme, type II 5-alpha reductase. This enzyme converts free testosterone into DHT, and then that DHT attaches to an androgen receptor in scalp tissues – thereby influencing cell function.
For these reasons, type II 5-alpha reductase and androgen receptors have been targets of both AGA research and AGA-related drugs. Finasteride is a type II 5-alpha reductase inhibitor; spironolactone and RU58841 are androgen receptor blockers. While these drugs don’t lead to complete AGA reversals, they do seem to improve AGA outcomes.
“DHT sensitivity” is a term used to describe how, in scalp tissues, DHT might begin to preferentially bind to androgen receptors – thereby having up to 5x greater influence over these cells’ behavior than other male hormones like testosterone.
While DHT is causally linked to AGA, it’s still unclear how this hormone causes hair follicle miniaturization. Research in female pattern hair loss brings to question DHT’s involvement in AGA – and suggests that in addition to androgens, other factors must be involved.
The hormone DHT is causally linked to AGA. Men who can’t produce DHT never go bald, DHT levels are higher in the scalps of balding men, and drugs that reduce scalp DHT help to stop AGA’s progression.
Scalp DHT is formed when free testosterone comes into contact with the enzyme, type II 5-alpha reductase. This enzyme converts free testosterone into DHT, and then that DHT attaches to an androgen receptor in scalp tissues – thereby influencing cell function.
For these reasons, type II 5-alpha reductase and androgen receptors have been targets of both AGA research and AGA-related drugs. Finasteride is a type II 5-alpha reductase inhibitor; spironolactone and RU58841 are androgen receptor blockers. While these drugs don’t lead to complete AGA reversals, they do seem to improve AGA outcomes.
“DHT sensitivity” is a term used to describe how, in scalp tissues, DHT might begin to preferentially bind to androgen receptors – thereby having up to 5x greater influence over these cells’ behavior than other male hormones like testosterone.
While DHT is causally linked to AGA, it’s still unclear how this hormone causes hair follicle miniaturization. Research in female pattern hair loss brings to question DHT’s involvement in AGA – and suggests that in addition to androgens, other factors must be involved.
Clinical Phenomenology
AGA is a continuous, protracted process, rather than a series of distinct disease phases, and each patient is characterized by a wide range of different features. With the progression of AGA, it behaves similarly to the process of tissue aging - in the hair follicles, as in other organs, the proportion of cells that carry only a structural function increases, sutures, constrictions, scars are formed, sclerosis occurs - an increased development of connective tissue components, which leads to a weakening of specific functions, thinning and hair loss
The shift of the frontal growth line back and baldness of the crown are the main signs of male androgenetic alopecia. In addition, areas of alopecia can merge into a single whole, as a result of which only a border of normal hair growth remains on the sides and on the back of the head of the scalp.
AGA can occur both in men and women, but it manifests differently between the two sexes.
In men, classic pattern balding appears as:
The shift of the frontal growth line back and baldness of the crown are the main signs of male androgenetic alopecia. In addition, areas of alopecia can merge into a single whole, as a result of which only a border of normal hair growth remains on the sides and on the back of the head of the scalp.
AGA can occur both in men and women, but it manifests differently between the two sexes.
In men, classic pattern balding appears as:
- Recession of the hairline at the temples
- Loss of hair at the vertex (crown)
Norwood-Hamilton Scale (Male Pattern Hair Loss)


AGA in women progresses more slowly, its severity is less, and shows a greater variety of responses to therapy.
There are three distinct patterns of AGA in women:
There are three distinct patterns of AGA in women:
- diffuse thinning of the crown area while maintaining the frontal hairline (Ludwig model);
- thinning and spreading to the central part of the scalp in violation of the frontal hairline (Christmas tree model);
- thinning associated with bitemporal bald patches (Hamilton model) is more common in menopausal women and in women with hyperandrogenism.
Ludwig Scale (Female Pattern Hair Loss)

Androgenetic alopecia in syndromes
In rare cases, extremely severe androgenetic alopecia or its early onset may be a symptom of a complex genetic disorder, such as:
- trichorinophalangeal syndrome;
- progeria;
- Laron syndrome;
- and myotonic dystrophy of Kurshman-Steinert-Batten.
Premature baldness
Androgenetic alopecia, the clinical signs of which appear between the ages of 10 and 20 years, is called premature or early baldness. In children before puberty, the disease, both in boys and girls, manifests itself exclusively as female pattern baldness.
The differential diagnosis for congenital hair loss includes:
The differential diagnosis for congenital hair loss includes:
- simple hypotrichosis;
- and ectodermal dysplasia (with concomitant physical/mental retardation, sweating, nail and tooth abnormalities).
Evolution of classifications

Determining the severity of androgenetic alopecia is challenging.
The top row shows four drawings carved on the walls of a primitive cave. It took about 30,000 years to classify the pattern of male hair loss shown in the bottom row. At present, various classification methods have been developed and modified.
The top row shows four drawings carved on the walls of a primitive cave. It took about 30,000 years to classify the pattern of male hair loss shown in the bottom row. At present, various classification methods have been developed and modified.
AHA classifications
Until recently, the Hamilton-Norwood classification (1951-1975) for men and the Ludwig classification (1977) for women, emphasizing gender heterogeneity, were the generally accepted standard.
Evolution or revolution?
There was a need to improve existing classifications, as a result of which a universal classification was created in 2007. In 2007 Lee et al. proposed a basic and specific (BASP) classification system, which is an improved version of the Norwood-Hamilton classification, includes four basic types (L, M, C, U) and two specific types (F and V).
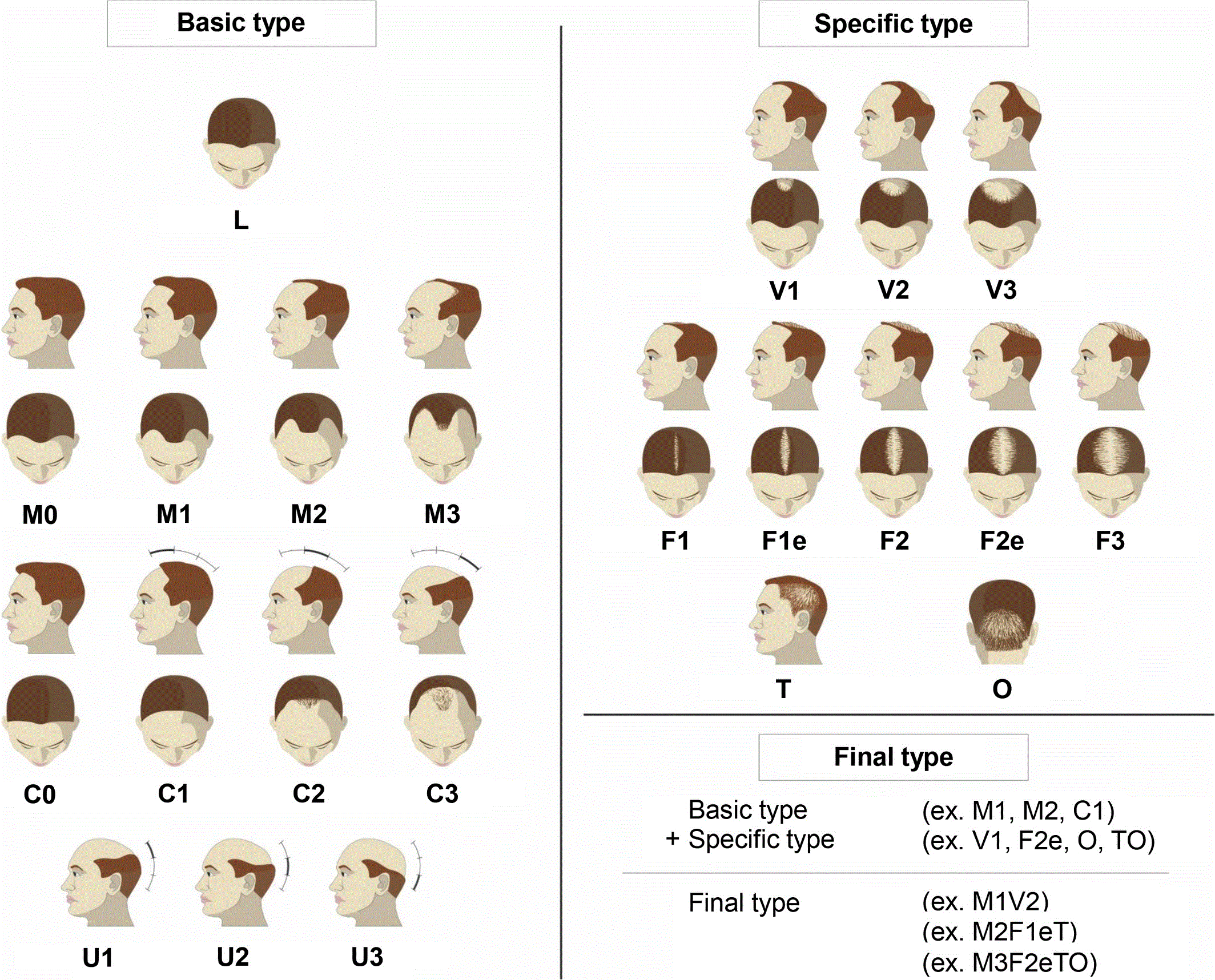
Anatomical regions of the scalp (a). The BAsic and SPecific classification systems (b). Four BAsic types (L, M, C and U) and two SPecific types (frontal [F] and vertex [V]). L, no recession is observed in the frontotemporal region; M, recession in the frontotemporal hairline is more obvious than the mid-anterior hairline; C, recession in the mid-anterior hairline is more obvious than the frontotemporal hairline; U, the anterior hairline recedes to the vertex forming a horseshoe shape.

Figure: Areas of the scalp. F-Frontal / M - Mid frontal / T-Temple / V-Vertex
The new pattern hair loss classification is a universal tool used for both men and women: the basic and specific (BASP) classification.
It can be used to evaluate both the further degree of hair loss and the response to therapy. By improving on the shortcomings of existing classifications, it is easy to remember and easier to apply in a clinical setting. The Norwood-Hamilton classification does not take into account some specific types of baldness, such as female pattern hair loss.
In addition, the Ludwig scale cannot be used to classify male pattern baldness in women. BASP classifies all types of hair loss regardless of gender or race.
It can be used to evaluate both the further degree of hair loss and the response to therapy. By improving on the shortcomings of existing classifications, it is easy to remember and easier to apply in a clinical setting. The Norwood-Hamilton classification does not take into account some specific types of baldness, such as female pattern hair loss.
In addition, the Ludwig scale cannot be used to classify male pattern baldness in women. BASP classifies all types of hair loss regardless of gender or race.


What are the hallmark characteristics of AGA?
AGA has specific features that distinguish it from other types of hair loss.
For starters, it primarily affects hair at the top part of the scalp – above the galea aponeurotica (the dense fibrous membrane that stretches across the top of the scalp).
Secondly, scalp regions affected by AGA show three defining characteristics of the condition:
Each of these phases are covered below.
For starters, it primarily affects hair at the top part of the scalp – above the galea aponeurotica (the dense fibrous membrane that stretches across the top of the scalp).
Secondly, scalp regions affected by AGA show three defining characteristics of the condition:
- Hair follicle miniaturization
- Increased telogen:anagen hairs
- Shortened anagen cycling
Each of these phases are covered below.
What is the normal hair growth cycle?
There are three types of hairs:
1. Lanugo hair. Fine long hair covering the foetus. Shed about 1 month before birth unless born prematurely. May reappear sometimes in severe malnutrition and anorexia nervosa.
2. Vellus hair. Fine, short unmedullated hair covering much of the body surface. They replace the lanugo hair just before birth.
3. Thick, pigmented hairs are called terminal hairs. Terminal hairs on the top of the head and in the beard, axillary, and bubic areas are influenced by androgenes.
1. Lanugo hair. Fine long hair covering the foetus. Shed about 1 month before birth unless born prematurely. May reappear sometimes in severe malnutrition and anorexia nervosa.
2. Vellus hair. Fine, short unmedullated hair covering much of the body surface. They replace the lanugo hair just before birth.
3. Thick, pigmented hairs are called terminal hairs. Terminal hairs on the top of the head and in the beard, axillary, and bubic areas are influenced by androgenes.


Figure: Schematic with possible dermal cellular mechanisms modulating a vellus-terminal transition. Increased influx into the dermal papilla (DP) during the telogen-anagen transition would trigger a large step change that would take a vellus hair to terminal state in one cycle. Comparatively, reduced DP cell efflux during catagen would over time result in a gradual increase in DP size assuming that influx of hfDSCs remains unchanged. Alternatively, both of these mechanisms may be required to act in tandem with one another to achieve a reversal of male pattern baldness
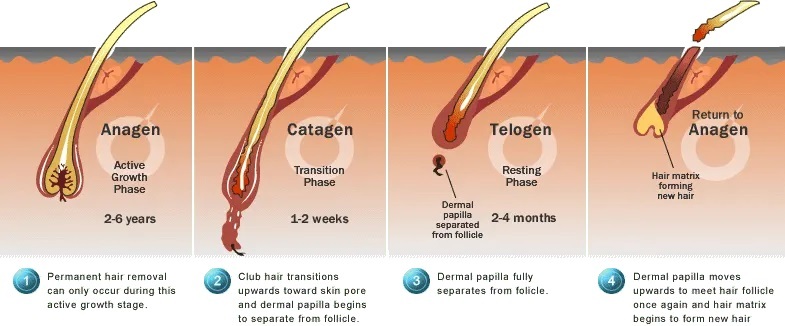
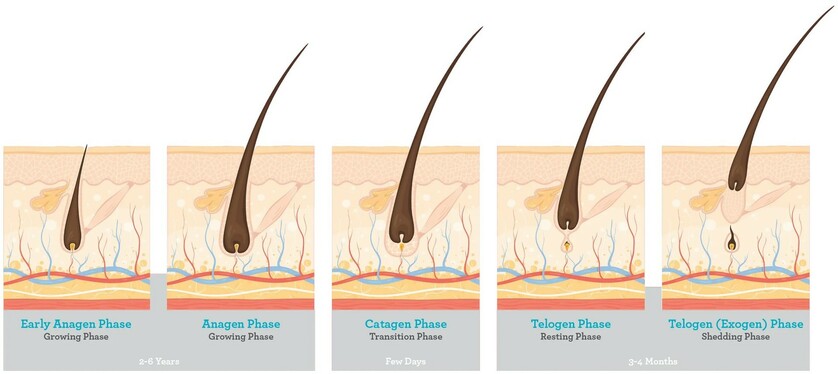
All hair follicles are replaced at different rates by the normal process of hair cycling. Hair growth alternates between phases of activity and rest.
1. Anagen Phase: The growth period, called the anagen phase, lasts for two to six years. During this time, the follicle is long and deep and produces thick, well-pigmented hair, the hair shaft is actively growing inside the follicle, with rapidly dividing new cells being supplied by the dermal papilla. About 90% of all scalp hairs are in the anagen phase at a given time.
2. Catagen Phase: Anagen is followed by a brief transition phase known as the catagen phase, which lasts 1–2 weeks. This is also called the degradation phase and is a transitional phase where the hair follicle pulls away from the dermal papilla. During this time, the base of the follicle shrivels. Since the hair follicle is no longer being supplied with the new cells from the dermal papilla, the hair stops growing. This stage is brief, usually lasting several days.
3. Telogen Phase: The resting period, or telogen phase, follows catagen and lasts for three months. In this phase, the shrunken follicle retains the hair fibre. Following the telogen phase, the next anagen phase begins, and the old hair is dislodged and falls out to make room for new hair to begin growing in its place. Since the hair follicle is no longer being supplied with the new cells from the dermal papilla, the hair stops growing. This lasts about 3-4 months.
(4). Exogen (shedding) phase: Exogen is a phase independent of the main hair follicle cycle where the club hair is actively shed from the old bulge. Exogen refers to the state of the club hair rather than the hair follicle.
(5). Kenogen (latent lag) phase: Kenogen is not observed in all hair follicles; however, frequency and duration increases in scalp follicles of individuals with AGA. It refers to a lag phase, or a follicle in telogen which has lost its club hair (exogen) prior to re-entry into anagen.
1. Anagen Phase: The growth period, called the anagen phase, lasts for two to six years. During this time, the follicle is long and deep and produces thick, well-pigmented hair, the hair shaft is actively growing inside the follicle, with rapidly dividing new cells being supplied by the dermal papilla. About 90% of all scalp hairs are in the anagen phase at a given time.
2. Catagen Phase: Anagen is followed by a brief transition phase known as the catagen phase, which lasts 1–2 weeks. This is also called the degradation phase and is a transitional phase where the hair follicle pulls away from the dermal papilla. During this time, the base of the follicle shrivels. Since the hair follicle is no longer being supplied with the new cells from the dermal papilla, the hair stops growing. This stage is brief, usually lasting several days.
3. Telogen Phase: The resting period, or telogen phase, follows catagen and lasts for three months. In this phase, the shrunken follicle retains the hair fibre. Following the telogen phase, the next anagen phase begins, and the old hair is dislodged and falls out to make room for new hair to begin growing in its place. Since the hair follicle is no longer being supplied with the new cells from the dermal papilla, the hair stops growing. This lasts about 3-4 months.
(4). Exogen (shedding) phase: Exogen is a phase independent of the main hair follicle cycle where the club hair is actively shed from the old bulge. Exogen refers to the state of the club hair rather than the hair follicle.
(5). Kenogen (latent lag) phase: Kenogen is not observed in all hair follicles; however, frequency and duration increases in scalp follicles of individuals with AGA. It refers to a lag phase, or a follicle in telogen which has lost its club hair (exogen) prior to re-entry into anagen.


Follicle Health & Hair Growth
Hair follicles are the foundation on which healthy strands of hair are grown. It is the follicle that supplies the root of the hair with the oxygen, nutrients, and support needed to flourish during the anagen phase. The follicle also plays a vital role in supporting hair as it transitions through the catagen phase. During telogen phase, the hair follicle rests in preparation for new hair growth.
Though much is still being discovered about the root causes of hair loss, research shows that many hair loss conditions are associated with certain conditions that impair hair follicle health and function. To illustrate, consider:
Though much is still being discovered about the root causes of hair loss, research shows that many hair loss conditions are associated with certain conditions that impair hair follicle health and function. To illustrate, consider:
- Telogen effluvium (stress-related hair loss) occurs after a stressful event causes enough trauma to “shock” the follicles into an inactive state. The hair loss condition receives its name from the telogen phase in which follicles become stuck.
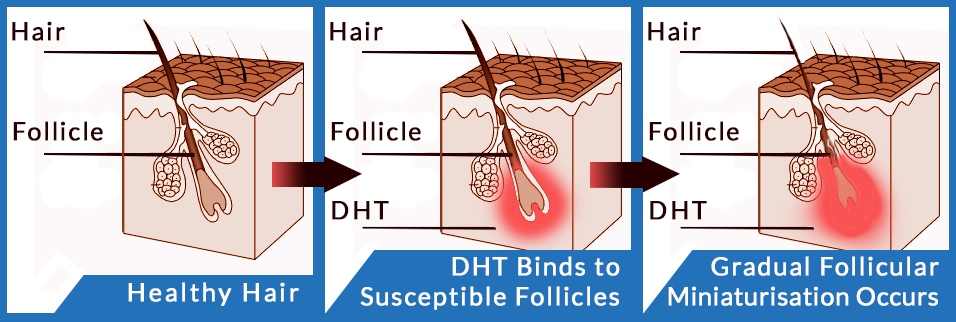
- Androgentic alopecia (pattern baldness) is correlated with high levels of DHT (the androgen dihydrotestosterone) on the scalp. DHT is thought to penetrate the scalp and cause hair follicle miniaturization, a phenomenon in which follicles become smaller and eventually incapable of supporting a healthy hair growth cycle.
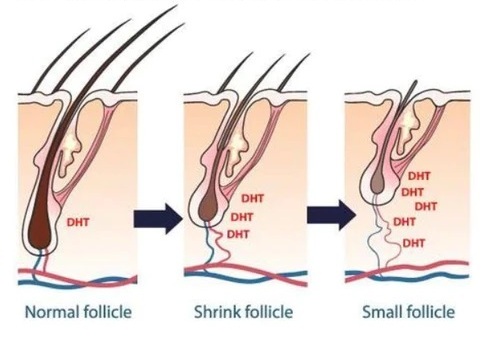
Hair follicle miniaturization
The development of AGA is based on gender homogeneity, which is associated with the genetic characteristics of androgen metabolism in the hair follicle.
Male pattern hair loss is an inherited condition, caused by a genetically determined sensitivity to the effects of dihydrotestosterone (DHT) in some areas of the scalp. DHT is believed to shorten the growth, or anagen, phase of the hair cycle, from a usual duration of 3–6 years to just weeks or months. Follicle miniaturization is unique to AGA. It is a process by which AGA-affected hair follicles progressively get smaller until they produce fewer hairs. The hairs that are left may transition into fine, wispy hairs – known as vellus hairs.
A few women present with male pattern hair loss because they have excessive levels of androgens as well as genetic predisposition. These women also tend to suffer from acne, irregular menses and excessive facial and body hair. These symptoms are characteristic of polycystic ovarian syndrome (PCOS) although the majority of women with PCOS do not experience hair loss. Less often, congenital adrenal hyperplasia may be responsible. In women, as a rule, a large role is played by a decrease in the activity of aromatase, which converts ovarian testosterone circulating in the blood into 17 beta-estradiol. Females that are losing their hair with age are more likely to present with female pattern hair loss, in which hormone tests are normal.
Male pattern hair loss is an inherited condition, caused by a genetically determined sensitivity to the effects of dihydrotestosterone (DHT) in some areas of the scalp. DHT is believed to shorten the growth, or anagen, phase of the hair cycle, from a usual duration of 3–6 years to just weeks or months. Follicle miniaturization is unique to AGA. It is a process by which AGA-affected hair follicles progressively get smaller until they produce fewer hairs. The hairs that are left may transition into fine, wispy hairs – known as vellus hairs.
A few women present with male pattern hair loss because they have excessive levels of androgens as well as genetic predisposition. These women also tend to suffer from acne, irregular menses and excessive facial and body hair. These symptoms are characteristic of polycystic ovarian syndrome (PCOS) although the majority of women with PCOS do not experience hair loss. Less often, congenital adrenal hyperplasia may be responsible. In women, as a rule, a large role is played by a decrease in the activity of aromatase, which converts ovarian testosterone circulating in the blood into 17 beta-estradiol. Females that are losing their hair with age are more likely to present with female pattern hair loss, in which hormone tests are normal.

An increase in the local concentration of DHT leads to a progressive contraction of the anaphase due to a longer telogen phase and is accompanied by a progressive miniaturization of the HF. Miniaturization of the HF occurs due to relatively sharp reductions in the number of cells in the dermal papilla and dermal membrane. Accompanied by:
While the causes and step-processes of hair follicle miniaturization are still debated, this process is often accompanied by histological (i.e. structural) changes – particularly to the tissues surrounding these hair follicles.
In fact, researchers believe these structural changes help to explain why pattern hair loss is a progressive condition – as they limit miniaturized hair follicles’ capabilities of returning back to full-size. They are:
All of these histological changes can be considered forms of scarring.
- reduction in the absolute number of HF,
- a decrease in the anaphase period,
- rod diameter,
- as well as an increase in the duration of the kenogen phase.
While the causes and step-processes of hair follicle miniaturization are still debated, this process is often accompanied by histological (i.e. structural) changes – particularly to the tissues surrounding these hair follicles.
In fact, researchers believe these structural changes help to explain why pattern hair loss is a progressive condition – as they limit miniaturized hair follicles’ capabilities of returning back to full-size. They are:
- Dermal sheath thickening
- Perifollicular fibrosis and perifollicular inflammation
- Oxidative stress
- The loss of connection between the levator pilus muscle and the follicular unit
- The replacement the levator pilus muscle by adipose tissue
- Increased telogen:anagen ratio
- Shortened anagen cycling
All of these histological changes can be considered forms of scarring.
Dermal sheat thickening and perifollicular fibrosis
Dermal sheath is a term used to describe the skin tissues that surround our hair follicles. Dermal sheaths are comprised of collagen (i.e., skin), blood vessels, sweat glands, lymphatic networks, and more.
In men and women with AGA, dermal sheaths surrounding miniaturizing hair follicles have thickened. Specifically, their collagen bundles are up to 2.5 times larger – a characteristic of early scar tissue development (Jaworsky et al., 1992).
As dermal sheaths thicken, they widen into the space occupied by hair follicles – thereby impeding their growth space. And as AGA progresses, these dense collagen bundles turn into scar tissue – also known as perifollicular fibrosis.
Together, dermal sheath thickening and perifollicular fibrosis restrict the growth space of surrounding hair follicles, which creates a spacial barrier for miniaturizing hair follicles to recover back to their original size.
In men and women with AGA, dermal sheaths surrounding miniaturizing hair follicles have thickened. Specifically, their collagen bundles are up to 2.5 times larger – a characteristic of early scar tissue development (Jaworsky et al., 1992).
As dermal sheaths thicken, they widen into the space occupied by hair follicles – thereby impeding their growth space. And as AGA progresses, these dense collagen bundles turn into scar tissue – also known as perifollicular fibrosis.
Together, dermal sheath thickening and perifollicular fibrosis restrict the growth space of surrounding hair follicles, which creates a spacial barrier for miniaturizing hair follicles to recover back to their original size.

Dermal sheath thickening is consistently observed in AGA. However, its advancement to perifollicular fibrosis has been found, according to some studies, in over 71% of AGA sufferers – with moderate levels observed in at least 37% (Whiting et al., 1996).
By progressively restricting the growth space of miniaturizing hair follicles, these histological changes also explain the chronic, progressive nature of AGA – along with why most treatments are limited mainly to stopping the progression of pattern hair loss rather than fully reversing it (English, 2018).
By progressively restricting the growth space of miniaturizing hair follicles, these histological changes also explain the chronic, progressive nature of AGA – along with why most treatments are limited mainly to stopping the progression of pattern hair loss rather than fully reversing it (English, 2018).
Hair follicle miniaturization is a hallmark of AGA. This is when the size of each hair follicle shrinks and thus starts producing smaller, wispier hairs.
This process is accompanied by dermal sheath thickening around miniaturizing hair follicles. This skin thickening is an early step-process of scarring. In later stages of AGA, this turns to perifollicular fibrosis.
Dermal sheath thickening and perifollicular fibrosis prevent AGA-affected hair follicles from rebounding to their full size. They help to explain the chronic, progressive nature of AGA.
This process is accompanied by dermal sheath thickening around miniaturizing hair follicles. This skin thickening is an early step-process of scarring. In later stages of AGA, this turns to perifollicular fibrosis.
Dermal sheath thickening and perifollicular fibrosis prevent AGA-affected hair follicles from rebounding to their full size. They help to explain the chronic, progressive nature of AGA.
The role of perifollicular inflammation
In addition to androgen-dependent changes in the pathogenesis of AGA, the involvement of follicular microinflammation with the formation of fibrosis provoked by the presence of bacterial flora, toxins and oxidative stress has been proven.
According to the obtained data, inflammatory markers such as fibrinogen, C-reactive protein and lipoprotein (a) acted as risk factors for cardiovascular diseases in AGA. AGA has also been shown to be associated with increased arterial stiffness, even in asymptomatic young adults.
While complex genetic inheritance and age of the individual are major risk factors in AGA development, on a cellular level, the initiation of an inflammatory condition in the follicle microenvironment is considered the central event, with contributory mechanisms, including abnormal signal transduction (the wingless-type integration site pathway), high levels of apoptosis, and oxidative stress.
DHT, a major factor causing hair loss in AGA, has been found to act downstream by inducing the production of interleukin- (IL-) 6 and transforming growth factor- (TGF-) β2 by dermal papilla cells (DPCs), thus suppressing hair growth and premature onset of the catagen phase.
According to the obtained data, inflammatory markers such as fibrinogen, C-reactive protein and lipoprotein (a) acted as risk factors for cardiovascular diseases in AGA. AGA has also been shown to be associated with increased arterial stiffness, even in asymptomatic young adults.
While complex genetic inheritance and age of the individual are major risk factors in AGA development, on a cellular level, the initiation of an inflammatory condition in the follicle microenvironment is considered the central event, with contributory mechanisms, including abnormal signal transduction (the wingless-type integration site pathway), high levels of apoptosis, and oxidative stress.
DHT, a major factor causing hair loss in AGA, has been found to act downstream by inducing the production of interleukin- (IL-) 6 and transforming growth factor- (TGF-) β2 by dermal papilla cells (DPCs), thus suppressing hair growth and premature onset of the catagen phase.
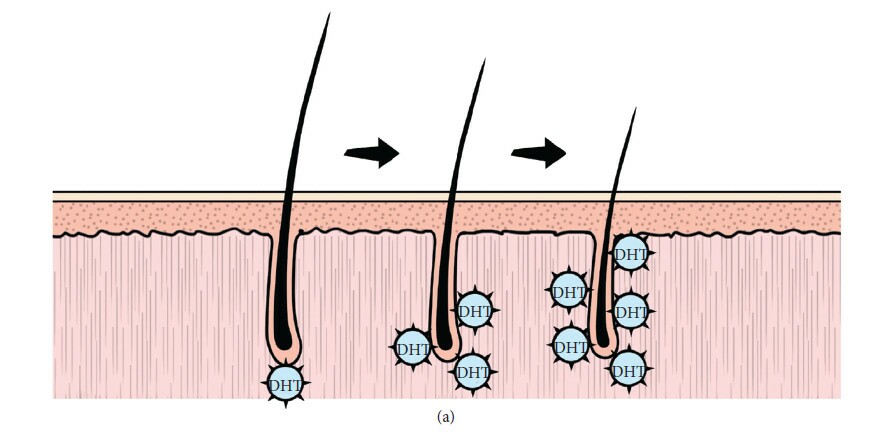

Figure: The hair follicle cycle showing the effects of various cytokines, growth promoters, and growth inhibitors regarding different stage transitions. BDNF, brain-derived neurotrophic factor; BMP-2/4, bone morphogenetic protein-2/4; col 17 A1, collagen-type XVII α 1 chain; DKK,Dickkopf-related protein; FGF5/7/18, fibroblast growth factor 5/7;GDNF,glial cellline-derived neurotrophic factor; HGF, hepatocyte growthfactor; IGF-1, insulin-like growth factor-1; IL-1/6, interleukin-6; KGF, keratinocyte growth factor; PG D2/E2/F2a, prostaglandin D2/ E2/F2a; SCF, stem cell factor; SHH, sonic hedgehog; TGF, transforming growth factor; TNF-α, tumor necrosis factor α; VDR=vitamin D receptor; WNT, wingless-type integration site.
Oxidative stress in the pathobiology of androgenetic alopecia
The search for mechanisms regulating the activation and progressive development of AGA continues. It has been found that oxidative stress is an important factor that contributes negatively to baldness.
Compared with cells taken from the occipital area without alopecia, cells of the dermal papilla from the balding area of men were characterized by a significantly greater sensitivity to oxidative stress. They also differed by reduced proliferation and migration, accompanied by an increase in the level of reactive oxygen species and aging.
Compared with cells taken from the occipital area without alopecia, cells of the dermal papilla from the balding area of men were characterized by a significantly greater sensitivity to oxidative stress. They also differed by reduced proliferation and migration, accompanied by an increase in the level of reactive oxygen species and aging.
The loss of connection between the levator pilus muscle and the follicular unit
Some experts consider AGA as an organ-specific segmental accelerated aging with increased sensitivity of hair follicle fibroblasts to oxidative stress.
Particular attention has recently been paid to the role of additional organs located in close proximity to the hair follicle. As a new participant in the pathogenesis of AGA, the levator pilus muscle was isolated.
Particular attention has recently been paid to the role of additional organs located in close proximity to the hair follicle. As a new participant in the pathogenesis of AGA, the levator pilus muscle was isolated.
It is hypothesized that the loss of connection between the levator pilus muscle and the follicular unit controls the miniaturization process in AGA and leads to its irreversibility, in contrast to the reversible process observed in alopecia areata, in which the connection between the muscle and the follicular unit is preserved.
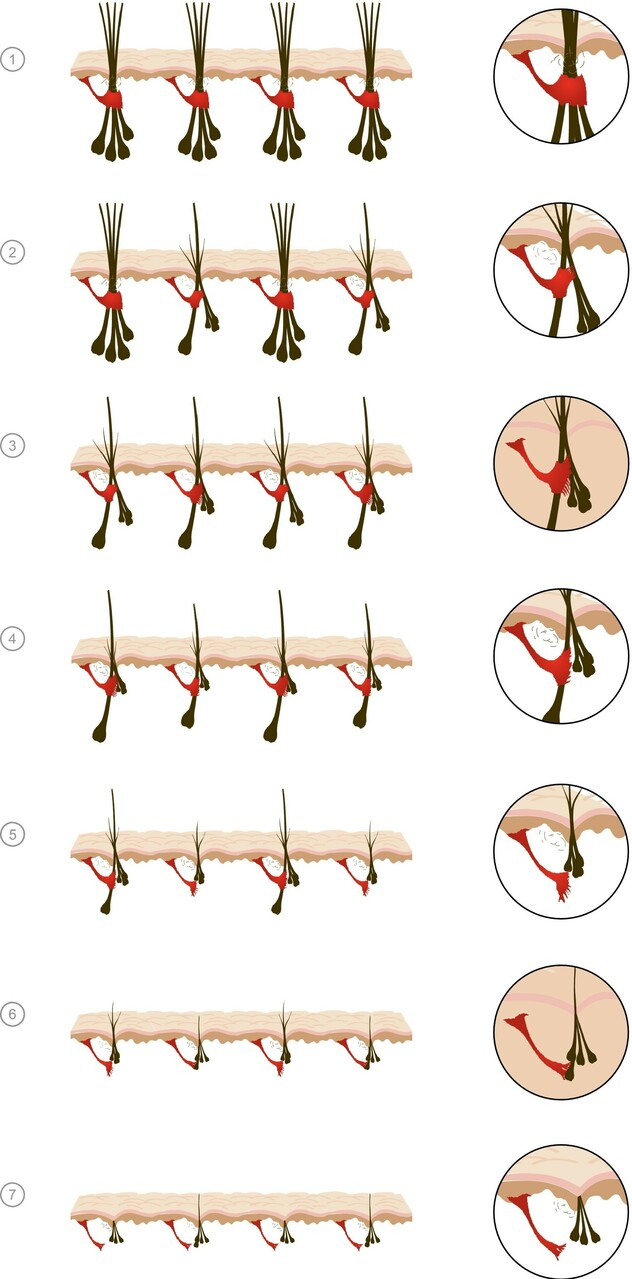
Figure: Progressive miniaturization within follicular units (FUs). Initially, multiple compound follicular units are found across the scalp comprising a primary follicle and multiple secondary follicles. Miniaturization occurs initially in the secondary follicles, leading to a reduction in hair density but no visible baldness. Baldness occurs when the entire follicular unit is miniaturized. As hairs miniaturize, there is progressive loss of contact between the APM and outer root sheath of the follicle at the level of the bulge. Loss of contact between the APM and ORS predicts irreversibility of miniaturization. In AGA, there appears to be a hierarchy of androgen sensitivity within FUs, the persisting terminal hairs represent primary follicle within the FU and that the miniaturized follicles that lose contact with the AP represent the secondary follicles.
The replacement the levator pilus muscle by adipose tissue
It has been suggested that the levator pilus muscle is replaced by adipose tissue, and this phenomenon may lead to depletion of stem or progenitor cells in the follicle mesenchyme.
Also, the sebaceous glands were put forward as potential factors in the pathology of AGA. It has been shown that in patients with AGA, the area of the sebaceous glands increases significantly with a simultaneous increase in the number of lobes in each follicular unit. It has been suggested that the close relationship between DHT and the functional activity of the sebaceous gland leads to their expansion, which can provoke an early transition to the catagen or telogen phase.
Androgen-dependent processes in target tissues occur mainly due to the binding of DHT to AR.
DHT-dependent cell functions are determined by the presence of weak androgens, their conversion into more powerful androgens under the action of 5-alpha-reductase, low enzymatic activity of androgen-deactivating enzymes, and the presence of large amounts of functionally active AR.
High levels of DHT and increased expression of AR were recorded on the scalp predisposed to the disease.
The conversion of testosterone to DHT in the dermal papilla plays a key role, while androgen-regulated factors produced by dermal papilla cells are thought to influence the growth of other components of the HF.
Only in a part of women with AGA, the presence of pathological changes in the metabolism of androgens in the serum of peripheral blood is confirmed, and deviations from the norm are often insignificant. In a Moltz study of 125 women with AGA, only 29% of free testosterone levels were elevated.
Described in AGA endocrine disorders on the other side of the androgen horizon, characterized by prolactinemia, impaired thyroid stimulating hormone (TSH), melatonin, somatotropin. There is an association with a decrease in serum ferritin (< 30 ng/mL).
Despite the variety of described hyperandrogenic conditions, more often AGA, both in women and men, occurs against the background of a normal level of androgens and is not accompanied by any endocrine disorders, the so-called receptor form.
Androgen-dependent processes in target tissues occur mainly due to the binding of DHT to AR.
DHT-dependent cell functions are determined by the presence of weak androgens, their conversion into more powerful androgens under the action of 5-alpha-reductase, low enzymatic activity of androgen-deactivating enzymes, and the presence of large amounts of functionally active AR.
High levels of DHT and increased expression of AR were recorded on the scalp predisposed to the disease.
The conversion of testosterone to DHT in the dermal papilla plays a key role, while androgen-regulated factors produced by dermal papilla cells are thought to influence the growth of other components of the HF.
Only in a part of women with AGA, the presence of pathological changes in the metabolism of androgens in the serum of peripheral blood is confirmed, and deviations from the norm are often insignificant. In a Moltz study of 125 women with AGA, only 29% of free testosterone levels were elevated.
Described in AGA endocrine disorders on the other side of the androgen horizon, characterized by prolactinemia, impaired thyroid stimulating hormone (TSH), melatonin, somatotropin. There is an association with a decrease in serum ferritin (< 30 ng/mL).
Despite the variety of described hyperandrogenic conditions, more often AGA, both in women and men, occurs against the background of a normal level of androgens and is not accompanied by any endocrine disorders, the so-called receptor form.
Increased telogen:anagen ratio
Beyond hair follicle miniaturization, the second defining characteristic of AGA is an increase in telogen versus anagen hairs.
Telogen and anagen are terms used to describe specific stages of the hair cycle.
The hair cycle is the natural, ever-repeating cycle of regeneration and degeneration of hair follicles. A hair grows, stops growing, and eventually falls out – at which point a new hair follicle reforms and a new hair starts growing again. In human scalps, these hair cycles last between two to seven years.
In non-balding scalps, 80-85% of scalp hairs are in their growth (anagen) phase, 1-2% have stopped growing and are in their resting (catagen) phase, and 10-15% have already fallen out and entered their shedding (telogen) phase.
In healthy scalps, this cycle repeats indefinitely. Thus, many hair loss disorders can sometimes be identified by measuring the number of shedding versus growing hairs – and then comparing that ratio to what is seen in normal scalps. If the ratio is high, this suggests abnormal hair loss. If it’s low, this suggests normal, healthy hair.
This is the telogen:anagen ratio.
In normal scalps, there is generally one telogen hair for every 12 anagen hairs, or a 1:12 ratio.
However, in men with AGA, the telogen:anagen ratio can exceed 1:4 (or 25%). In women, this ratio often exceeds 2:5 (or 40%).
Telogen and anagen are terms used to describe specific stages of the hair cycle.
The hair cycle is the natural, ever-repeating cycle of regeneration and degeneration of hair follicles. A hair grows, stops growing, and eventually falls out – at which point a new hair follicle reforms and a new hair starts growing again. In human scalps, these hair cycles last between two to seven years.
In non-balding scalps, 80-85% of scalp hairs are in their growth (anagen) phase, 1-2% have stopped growing and are in their resting (catagen) phase, and 10-15% have already fallen out and entered their shedding (telogen) phase.
In healthy scalps, this cycle repeats indefinitely. Thus, many hair loss disorders can sometimes be identified by measuring the number of shedding versus growing hairs – and then comparing that ratio to what is seen in normal scalps. If the ratio is high, this suggests abnormal hair loss. If it’s low, this suggests normal, healthy hair.
This is the telogen:anagen ratio.
In normal scalps, there is generally one telogen hair for every 12 anagen hairs, or a 1:12 ratio.
However, in men with AGA, the telogen:anagen ratio can exceed 1:4 (or 25%). In women, this ratio often exceeds 2:5 (or 40%).
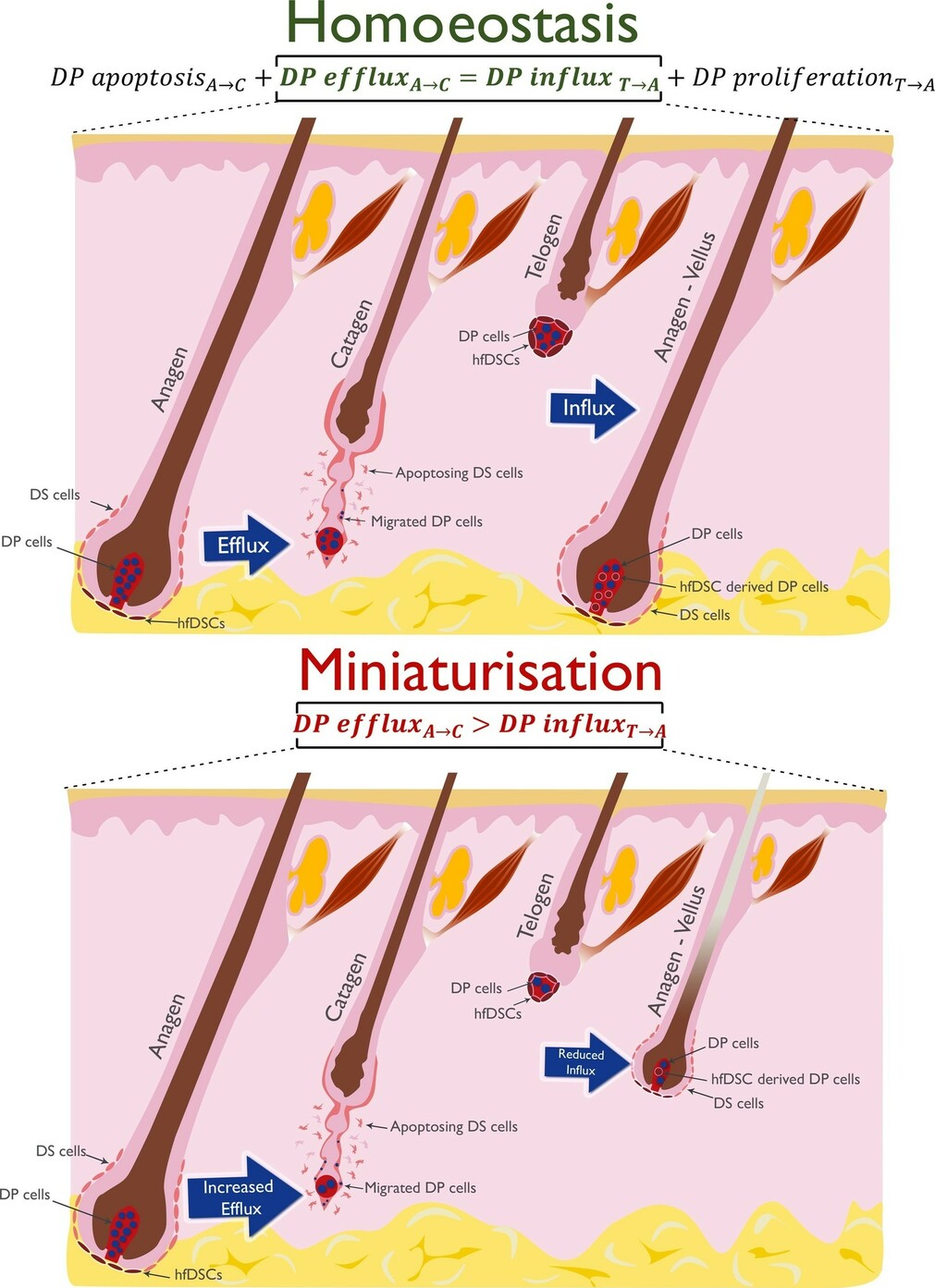
Figure: Simplified dermal mechanisms underlying terminal follicle cycling and miniaturisation. In catagen, dermal papilla (DP) cells migrate out of the DP into the dermal sheath (DS) which is largely degraded. In telogen, the dermal compartment of the hair follicle is reduced with a small number of hair dermal follicle stem cells (hfDSC) surrounding the DP. In homoeostasis, the DP is fully restored via proliferation and migration of these hfDSC into the DP during anagen resulting in the same number of cells in the DP as in the previous anagen. In miniaturisation, increased efflux of DP cells may occur in the anagen to catagen transition. Alternatively, or conjointly, hfDSCs might not replenish the DP fully in the telogen to anagen transition. Loss of hair follicle homoeostasis may be due to external factors such as DHT and/or perturbations to self-regulating modulators.
Shortened anagen cycling
Moreover, in balding scalps, the anagen phase of a miniaturizing hair is shortened. This means that rather than growing for 2-7 years, these hairs may only grow for a few months (Ho et al., 2019).
This is why so many AGA-affected men and women notice short terminal hairs near their hairline or vertex – hairs that won’t grow more than a few inches. This is the result of a shortened anagen phase and an increased telogen:anagen ratio.
This is why so many AGA-affected men and women notice short terminal hairs near their hairline or vertex – hairs that won’t grow more than a few inches. This is the result of a shortened anagen phase and an increased telogen:anagen ratio.
In AGA, balding regions have an elevated ratio of shedding to growing hairs. This is called the telogen:anagen ratio.
While healthy scalps have a telogen:anagen ratio of 10-15%, balding regions will have a ratio of 25-40%, and sometimes higher.
Moreover, growing (anagen) hairs in balding regions often do not grow for more than a few months. This is called a shortened anagen phase. This helps to explain why so many balding men and women see shorter hairs in balding regions that never reach more than an inch or two in length.
While healthy scalps have a telogen:anagen ratio of 10-15%, balding regions will have a ratio of 25-40%, and sometimes higher.
Moreover, growing (anagen) hairs in balding regions often do not grow for more than a few months. This is called a shortened anagen phase. This helps to explain why so many balding men and women see shorter hairs in balding regions that never reach more than an inch or two in length.
Relationship with other states
Research published over the past few years has further strengthened the link between AGA and CVD risk and metabolic syndrome. Separately, it is emphasized that these diseases are more consistent with AGA of early onset, affecting the crown area.
In addition, AGA has been shown to be an independent predictor of death from diabetes mellitus and cardiovascular disease.
The association between AGA and benign prostatic hyperplasia (BPH) has also been reinforced. It has been shown that patients suffering from BPH with inflammation of the prostate gland are distinguished by a higher severity of AGA.
An association was also found between prostatic hyperplasia (BPH), AGA, and AR gene polymorphism (SNP rs6152 G>A).
According to a recent meta-analysis, crown spread of AGA is associated with a significant increase in prostate cancer risk, while other types of AGA spread do not show a significant increase in prostate cancer risk.
In addition, AGA has been shown to be an independent predictor of death from diabetes mellitus and cardiovascular disease.
The association between AGA and benign prostatic hyperplasia (BPH) has also been reinforced. It has been shown that patients suffering from BPH with inflammation of the prostate gland are distinguished by a higher severity of AGA.
An association was also found between prostatic hyperplasia (BPH), AGA, and AR gene polymorphism (SNP rs6152 G>A).
According to a recent meta-analysis, crown spread of AGA is associated with a significant increase in prostate cancer risk, while other types of AGA spread do not show a significant increase in prostate cancer risk.
What Causes Postpartum Hair Loss?
During pregnancy, estrogen levels are increased, which lengthens the hair growth phase and lessens the shedding phase, resulting in a thicker than normal head of hair. After pregnancy (postpartum), estrogen levels fall, resulting in an increased shedding/resting phase (telogen). This also results in less hair in the growth phase (anagen). Postpartum hair loss is temporary and normal, with hair growth cycles normalizing six months to a year after pregnancy and restoring your hair to pre-pregnancy thickness.
What Causes Hair Loss in Menopause?
The hormonal changes of menopause such as the decrease of estrogen and progesterone causes a hormonal imbalance that can result in hair loss. However, it is recommended that hormone replacement therapy be closely supervised. Other factors like nutrition, illness, and medications are increased during menopause, and can also be contributing to hair loss.
Can Stress Cause Hair to Fall Out?
Sudden and severe stress can slow new hair growth, then followed by a delayed hair shedding (telogen) phase. This results in a higher than normal amount of hair entering the shedding phase at the same time, resulting in visible patches of temporary hair loss. This is scientifically known as telogen effluvium.
What don’t we know about AGA?
Despite AGA’s prevalence and decades of study, there are still a lot of unknowns about this hair loss disorder.
The specific genetics involved
As mentioned, AGA is a polygenic disorder. It has been unequivocally established that male pattern baldness is more likely to occur in men whose fathers suffer from AGA.
However, recent evidence suggests that genetic variances in the gene that encodes for androgen receptors are prevalent among men with male pattern baldness (Ellis et al., 2001). Because the androgen receptor gene is located on the X chromosome, which inherited by men from their mother, this raises questions about whether or not AGA is strictly related to paternal genetics.
Even more confounding is female AGA. Of the genetic components explore in male AGA, none seem to be present in women suffering from pattern hair loss (Ramos et al., 2015).
However, some research has demonstrated is that female pattern hair loss may be associated with polymorphisms in the aromatase gene (aromatase helps synthesize estrogen). Aromatase enzymes help convert testosterone into estrogen. However, it’s still unclear how (or why) these polymorphisms impact both female pattern hair loss – especially as this hair loss disorder has been characterized as “androgenic” since the 1970’s.
From the current body of evidence, it’s almost impossible to distinguish a specific gene or set of genes that is indisputably linked to all cases of AGA.
However, recent evidence suggests that genetic variances in the gene that encodes for androgen receptors are prevalent among men with male pattern baldness (Ellis et al., 2001). Because the androgen receptor gene is located on the X chromosome, which inherited by men from their mother, this raises questions about whether or not AGA is strictly related to paternal genetics.
Even more confounding is female AGA. Of the genetic components explore in male AGA, none seem to be present in women suffering from pattern hair loss (Ramos et al., 2015).
However, some research has demonstrated is that female pattern hair loss may be associated with polymorphisms in the aromatase gene (aromatase helps synthesize estrogen). Aromatase enzymes help convert testosterone into estrogen. However, it’s still unclear how (or why) these polymorphisms impact both female pattern hair loss – especially as this hair loss disorder has been characterized as “androgenic” since the 1970’s.
From the current body of evidence, it’s almost impossible to distinguish a specific gene or set of genes that is indisputably linked to all cases of AGA.
What causes DHT to increase in balding scalp regions?
If DHT is elevated in balding scalps of most men (and some women), what causes DHT to increase in these regions? This is one question researchers are still trying to answer.
One small-scale study indicates that whole-body DHT may not be the culprit – or that AGA patients don’t seem to have elevated serum DHT versus controls (Urysiak-Czubatka et al., 2014). This gives the impression that elevated DHT in balding scalp tissue is likely a local issue, and not a systemic problem.
So, then, what causes DHT to increase in scalp tissues, specifically? Elevated 5α-reductase activity has been implicated, which explains the ability of 5α-reductase inhibitors like finasteride to halt the progression of AGA (English, 2018). However, finasteride treatment doesn’t fully reverse AGA; it generally just stop its progression.
Findings that AGA is not associated with polymorphisms in the 5α-reductase genes suggest that this enzymatic upregulation is unrelated to genetic predisposition (Ellis et al., 1998). This indicates a possible environmental factor. This assumption is supported by studies showing that in genetically identical twins, one twin can bald faster than his counterpart – despite both twins having the same sets of genes (Nyholt et al., 2003).
Although research is sparse regarding environmental factors influencing 5α-reductase activity, there are some clues.
In women with PCOS, insulin resistance is related to increased 5α-reductase activity, possibly explaining why pattern hair loss is often a feature of the disorder (Wu et al., 2017). Male AGA is also associated with insulin resistance, however, whether this is a result of enhanced enzyme activity is unclear (González-González et al., 2009).
But again, no research team has discovered a definitive answer to this question.
One small-scale study indicates that whole-body DHT may not be the culprit – or that AGA patients don’t seem to have elevated serum DHT versus controls (Urysiak-Czubatka et al., 2014). This gives the impression that elevated DHT in balding scalp tissue is likely a local issue, and not a systemic problem.
So, then, what causes DHT to increase in scalp tissues, specifically? Elevated 5α-reductase activity has been implicated, which explains the ability of 5α-reductase inhibitors like finasteride to halt the progression of AGA (English, 2018). However, finasteride treatment doesn’t fully reverse AGA; it generally just stop its progression.
Findings that AGA is not associated with polymorphisms in the 5α-reductase genes suggest that this enzymatic upregulation is unrelated to genetic predisposition (Ellis et al., 1998). This indicates a possible environmental factor. This assumption is supported by studies showing that in genetically identical twins, one twin can bald faster than his counterpart – despite both twins having the same sets of genes (Nyholt et al., 2003).
Although research is sparse regarding environmental factors influencing 5α-reductase activity, there are some clues.
In women with PCOS, insulin resistance is related to increased 5α-reductase activity, possibly explaining why pattern hair loss is often a feature of the disorder (Wu et al., 2017). Male AGA is also associated with insulin resistance, however, whether this is a result of enhanced enzyme activity is unclear (González-González et al., 2009).
But again, no research team has discovered a definitive answer to this question.
How, exactly, does DHT miniaturize hair follicles?
No one is sure. The closest answers we have (so far) come from DHT and its link to a signaling protein called transforming growth factor beta 1 (TGF-β1).
Follicular miniaturization, as well as dermal sheath thickening and perifollicular fibrosis, are key features of AGA. In vitro studies strongly implicate the role of a growth factor, TGF-β1, in these processes (Yoo et al., 2006).
One of TGF-β1’s primary roles in the body is promoting both wound healing and the deposition of fibrous scar tissue (Pakyari et al., 2013). Thus, elevated TGF-β1 activity is likely involved in AGA follicle miniaturization and may even contribute to the process.
TGF-β1 expression can be triggered by the binding of androgens – like DHT – to androgen receptors, and in a potentially dose-dependent manner (Yoo et al., 2006). TGF-β1 may also enhance androgen activity through an androgen co-activator, Hic-5, that allows androgens like DHT to more effectively influence gene expression, like TGF-β1, within cells (Dabiri et al., 2008).
Essentially, increased androgen activity enhances TGF-β1 expression, and TGF-β1 exacerbates androgen activity. This feedback loop creates a vicious cycle that may underpin AGA progression.
Genetically-determined increased androgen receptor expression in AGA tissue contributes to this upregulated androgen activity, but this genetic component alone isn’t enough to dictate AGA progression (Nyholt et al., 2003).
This begs the question: in AGA, what causes androgen activity to increase?
If activity is dictated by androgen availability in the hair follicle, the rate of DHT conversion by 5α-reductase (which is controlled by the number of 5α-reductase enzymes and their activity), and the involvement of co-activators, then what, beyond genetics, could trigger any one of these factors?
Future research will hopefully begin to answer these questions.
Follicular miniaturization, as well as dermal sheath thickening and perifollicular fibrosis, are key features of AGA. In vitro studies strongly implicate the role of a growth factor, TGF-β1, in these processes (Yoo et al., 2006).
One of TGF-β1’s primary roles in the body is promoting both wound healing and the deposition of fibrous scar tissue (Pakyari et al., 2013). Thus, elevated TGF-β1 activity is likely involved in AGA follicle miniaturization and may even contribute to the process.
TGF-β1 expression can be triggered by the binding of androgens – like DHT – to androgen receptors, and in a potentially dose-dependent manner (Yoo et al., 2006). TGF-β1 may also enhance androgen activity through an androgen co-activator, Hic-5, that allows androgens like DHT to more effectively influence gene expression, like TGF-β1, within cells (Dabiri et al., 2008).
Essentially, increased androgen activity enhances TGF-β1 expression, and TGF-β1 exacerbates androgen activity. This feedback loop creates a vicious cycle that may underpin AGA progression.
Genetically-determined increased androgen receptor expression in AGA tissue contributes to this upregulated androgen activity, but this genetic component alone isn’t enough to dictate AGA progression (Nyholt et al., 2003).
This begs the question: in AGA, what causes androgen activity to increase?
If activity is dictated by androgen availability in the hair follicle, the rate of DHT conversion by 5α-reductase (which is controlled by the number of 5α-reductase enzymes and their activity), and the involvement of co-activators, then what, beyond genetics, could trigger any one of these factors?
Future research will hopefully begin to answer these questions.
Why is DHT also associated with body and facial hair growth?
DHT is linked to hair loss in AGA scalps. But ironically, this same hormone also enhances facial and body hair growth (Thornton et al.,1993). This occurs in spite of a 3-5 times higher 5α-reductase activity in beard follicles.
Given androgens and their negative impact on hair growth in the scalp, one would assume that elevated 5α-reductase activity would result in beard hair loss, not growth. But, this isn’t true for either case. Why might this be?
Given androgens and their negative impact on hair growth in the scalp, one would assume that elevated 5α-reductase activity would result in beard hair loss, not growth. But, this isn’t true for either case. Why might this be?
AGA hypothesis
No one is sure. Initially, researchers thought the pattern of AGA was due to different levels of androgen activity in balding regions. However, anecdotes of females with AGA and no androgen activity have raised questions about whether this actually makes sense.
In AGA scalps, there are differences in androgen receptor density and 5α-reductase activity in balding and non-balding regions (Cranwell et al., 2016). This may be explained by in vitro research that proposes individual follicles can “self-regulate”, modulating levels of androgen receptors and 5α-reductase enzymes.
Another question is then: what triggers these follicles to self-regulate? The current state of evidence leaves the answers to these questions purely speculative.
The activation of androgen co-activator, Hic-5, also increases follicle sensitivity to androgens, allowing androgens to more effectively influence hair loss-related gene expression (Tellez-Segura, 2015). Interestingly, stretching forces on follicle cells and oxidative stress have been shown to activate Hic-5 (Kim-Kaneyama et al., 2005, Shibanuma et al., 2003).
Elevated levels of reactive oxygen species (ROS, also known as free radicals) and increased stretching forces in these localized regions may explain increased androgen sensitivity, TGF-β activity, and, thus, the patterning of AGA.
What is not known is what events may trigger increased tension or free radical concentration in these areas. There’s still much to be explored.
In AGA scalps, there are differences in androgen receptor density and 5α-reductase activity in balding and non-balding regions (Cranwell et al., 2016). This may be explained by in vitro research that proposes individual follicles can “self-regulate”, modulating levels of androgen receptors and 5α-reductase enzymes.
Another question is then: what triggers these follicles to self-regulate? The current state of evidence leaves the answers to these questions purely speculative.
The activation of androgen co-activator, Hic-5, also increases follicle sensitivity to androgens, allowing androgens to more effectively influence hair loss-related gene expression (Tellez-Segura, 2015). Interestingly, stretching forces on follicle cells and oxidative stress have been shown to activate Hic-5 (Kim-Kaneyama et al., 2005, Shibanuma et al., 2003).
Elevated levels of reactive oxygen species (ROS, also known as free radicals) and increased stretching forces in these localized regions may explain increased androgen sensitivity, TGF-β activity, and, thus, the patterning of AGA.
What is not known is what events may trigger increased tension or free radical concentration in these areas. There’s still much to be explored.
The current model of AGA doesn’t fully explain (1) what causes DHT to increase in balding regions, (2) how DHT actually miniaturizes hair follicles, (3) why DHT is associated with scalp hair loss, but body and facial hair growth, and (4) why there is a unique pattern and progression to AGA.
Where is AGA research heading?
As scientists try to answer these questions, they’re beginning to stumble into new (and exciting) areas of AGA research.
Wnt/β-catenin signaling
The hair cycle is a degenerative and regenerative process that requires stem cells. Without the activity of these stem cells, the hair follicle can’t properly regenerate hair or maintain the integrity of the hair follicle itself.
Each compartment of the hair follicle possesses its own stem cell reservoir from which it draws. They are used to repair the epidermis around the hair follicle following injury, maintain the structure of the hair follicle, and help regulate hair follicle cycling (Yang et al., 2010).
Wnt/β-catenin signaling is essential for this process. These pathways help with the differentiation of stem cells in and around the hair follicle.
If this signaling pathway is blocked, hair follicle shaft regeneration is impaired. Blockade of this pathway may also impede the re-entry to the anagen phase from telogen.
In AGA, elevated levels of DHT effectively stimulate androgen-related gene transcription. One of the genes that androgens regulate is dickkopf 1 (DKK-1), a direct inhibitor of the Wnt/β-catenin pathway (Kwack et al., 2008).
When DHT binds to ARs in the dermal papilla cells, it enhances the expression and secretion of DKK-1. It acts in a paracrine fashion, meaning it affects hair components outside of the dermal papilla and may impair stem cell function throughout the hair follicle.
As a result, the hair follicle loses its integrity and may become inactive. However, evidence from animal studies indicates that, thankfully, even long-term DKK-1 inhibition of hair growth may be reversible (Choi et al., 2014).
Each compartment of the hair follicle possesses its own stem cell reservoir from which it draws. They are used to repair the epidermis around the hair follicle following injury, maintain the structure of the hair follicle, and help regulate hair follicle cycling (Yang et al., 2010).
Wnt/β-catenin signaling is essential for this process. These pathways help with the differentiation of stem cells in and around the hair follicle.
If this signaling pathway is blocked, hair follicle shaft regeneration is impaired. Blockade of this pathway may also impede the re-entry to the anagen phase from telogen.
In AGA, elevated levels of DHT effectively stimulate androgen-related gene transcription. One of the genes that androgens regulate is dickkopf 1 (DKK-1), a direct inhibitor of the Wnt/β-catenin pathway (Kwack et al., 2008).
When DHT binds to ARs in the dermal papilla cells, it enhances the expression and secretion of DKK-1. It acts in a paracrine fashion, meaning it affects hair components outside of the dermal papilla and may impair stem cell function throughout the hair follicle.
As a result, the hair follicle loses its integrity and may become inactive. However, evidence from animal studies indicates that, thankfully, even long-term DKK-1 inhibition of hair growth may be reversible (Choi et al., 2014).
Current Model
Alternative Model
How do androgens cause hair loss?
Genetic predispositions
Increased androgen activity and genetic sensitivity work synergistically to inhibit Wnt/β-catenin signaling, preventing hair follicle regeneration and anagen re-entry.
Galea interaction
The galea aponeurotica is a fibrous connective tissue that extends over the top of the scalp. It is attached to and connects all the muscles surrounding it.
The galea is a key component of the scalp tension theory of AGA. Interestingly, the “pattern” observed in AGA directly correlates to the highest points of tension in the GA (English, 2018).
Hair follicles are fashioned within both the dermis and fatty tissue beneath the skin, which is directly fused with the GA. Tension in the galea may be transmitted to these tissues and the follicles that are housed within.
Stretch-induced mechanical tension can enhance free radical production and trigger chronic inflammation in various types of tissues, potentially including the GA and the tissues that surround it. Cellular mechanical tension, free radicals, and inflammation can all enhance androgen activity and the fibrosis-mediating TGF-β1 (English, 2018).
This may, in part, explain the origin of follicle miniaturization in AGA.
Other research groups have developed similar hypotheses, arguing that fibrosis may not only drive hair follicle miniaturization, but that this process is potentially mediated by interactions between the galea and cells that transition adipose (fat) tissue into scar tissue (Kruglikov et al., 2017).
The galea is a key component of the scalp tension theory of AGA. Interestingly, the “pattern” observed in AGA directly correlates to the highest points of tension in the GA (English, 2018).
Hair follicles are fashioned within both the dermis and fatty tissue beneath the skin, which is directly fused with the GA. Tension in the galea may be transmitted to these tissues and the follicles that are housed within.
Stretch-induced mechanical tension can enhance free radical production and trigger chronic inflammation in various types of tissues, potentially including the GA and the tissues that surround it. Cellular mechanical tension, free radicals, and inflammation can all enhance androgen activity and the fibrosis-mediating TGF-β1 (English, 2018).
This may, in part, explain the origin of follicle miniaturization in AGA.
Other research groups have developed similar hypotheses, arguing that fibrosis may not only drive hair follicle miniaturization, but that this process is potentially mediated by interactions between the galea and cells that transition adipose (fat) tissue into scar tissue (Kruglikov et al., 2017).
Current Model
Alternative Model
What causes increased androgen activity in AGA follicles?
Genetic predisposition to androgen sensitivity.
Mechanical stretch, free radical production, and chronic inflammation as a result of galea tension enhance androgen activity.
Why is there increased androgen activity in localized regions?
Genetics don’t yet offer any direct explanation.
The points of highest tension within the galea directly correlate to the pattern seen in AGA. The mechanical stretch, free radical production, and chronic inflammation may all upregulate androgen activity.
Why does DHT cause hair loss on the scalp but not in facial or body hair?
Genetics don’t yet offer any explanation.
Mechanical stretch, free radical production, and chronic inflammation as a result of GA tension trigger TGF-β1 expression that leads to follicle miniaturization.
Olfactory receptors
An interesting finding of one 2018 study was the identification of OR2AT4 receptors in human hair follicles. OR2AT4 is an olfactory or smell receptor that is activated by certain scents (Chéret, et al. 2018).
In this study, researchers demonstrated that activation of this receptor by a synthetic sandalwood scent prolonged the anagen phase of the hair cycle. In fact, their findings suggest that activation of OR2AT4 may actually be indispensable for maintaining anagen.
While OR2AT4 is primarily considered an olfactory receptor activated by scent, this study also suggests that it may be activated by other compounds such as scalp microflora (bacteria) metabolites and short-chain fatty acids.
In this study, researchers demonstrated that activation of this receptor by a synthetic sandalwood scent prolonged the anagen phase of the hair cycle. In fact, their findings suggest that activation of OR2AT4 may actually be indispensable for maintaining anagen.
While OR2AT4 is primarily considered an olfactory receptor activated by scent, this study also suggests that it may be activated by other compounds such as scalp microflora (bacteria) metabolites and short-chain fatty acids.
Current Model
Alternative Model
Inhibition of androgen activity is the best way to treat AGA.
Many different cellular receptors in the hair follicle, like olfactory receptors, may help counteract the effects of androgens in the scalp.
In addition to PPAR pathways and retinoid receptors – Wnt-β catenin signaling, galea interactions, and olfactory receptors may help explain much of the unexplained phenomena in AGA pathology.
Diagnosis
Laboratory studies
History and the physical examination are the most important aspects of diagnosis in patients with androgenetic alopecia. The following laboratory tests, however, can play a role in patient assessment:
- Dehydroepiandrosterone (DHEA)-sulfate and testosterone analysis: In women, if virilization is evident
- Iron, total iron-binding capacity, and transferrin saturation: To test for iron deficiency, if telogen effluvium is present
- Thyrotropin level: If a thyroid disorder is suspected
Biopsy and histology
A biopsy is rarely necessary to make the diagnosis of androgenetic alopecia. If a single biopsy specimen is obtained, it should generally be sectioned transversely if pattern alopecia is suspected.
In androgenetic alopecia, hairs are miniaturized. Although the condition is considered a noninflammatory form of hair loss, a superficial, perifollicular, inflammatory infiltrate is noted at times. A mildly increased telogen-to-anagen ratio is often observed.
In androgenetic alopecia, hairs are miniaturized. Although the condition is considered a noninflammatory form of hair loss, a superficial, perifollicular, inflammatory infiltrate is noted at times. A mildly increased telogen-to-anagen ratio is often observed.
What are the treatment targets for AGA?
Most AGA treatments target to (1) decrease the telogen:anagen ratio, and (2) stop the progression of hair follicle miniaturization.
There are many ideas as to how to do this, but the most successful FDA-approved approach (so far) seems to be finasteride (Propecia®), a type II 5-alpha reductase inhibitor.
1mg daily of oral finasteride reduces scalp DHT levels by 50-70%. Subsequently, it stops AGA progression in 80-90% of men and leads to a 10% increase in hair count over two years, along with some additional hair thickening (English, 2018).
However, prolonged finasteride use is sometimes associated with sexual side effects. And, when considering all of the evidence that not just androgens are involved in AGA, it’s likely that finasteride is not a complete solution.
As such, here’s a list of current treatment targets in AGA research. Many of these targets overlap with one another.
In the years to come, this list will undoubtedly grow as researchers elucidate more of the molecular mechanisms behind AGA.
A suggested algorithm for therapeutic management of AGA is shown in Figure lower.
There are many ideas as to how to do this, but the most successful FDA-approved approach (so far) seems to be finasteride (Propecia®), a type II 5-alpha reductase inhibitor.
1mg daily of oral finasteride reduces scalp DHT levels by 50-70%. Subsequently, it stops AGA progression in 80-90% of men and leads to a 10% increase in hair count over two years, along with some additional hair thickening (English, 2018).
However, prolonged finasteride use is sometimes associated with sexual side effects. And, when considering all of the evidence that not just androgens are involved in AGA, it’s likely that finasteride is not a complete solution.
As such, here’s a list of current treatment targets in AGA research. Many of these targets overlap with one another.
- Type II 5-alpha reductase
- Androgen receptors
- Reactive oxygen species
- Wnt-β catenin pathways
- DKK-1
- TGF-β1
- Prostaglandin analogues
- Olfactory receptors
- PPAR pathways
- Retinoid receptors
- Potassium ion channels
- Mechanical tension
- Adipose-myofibroblast transitions
- Scalp microflora
In the years to come, this list will undoubtedly grow as researchers elucidate more of the molecular mechanisms behind AGA.
A suggested algorithm for therapeutic management of AGA is shown in Figure lower.

Here are some drug free options to block dihydrotestosterone:
Essential oils:
- Dong Quai
- Red Clover
- Black Cohosh
- Saw Palmetto
- Pumpkin Seed Oil
- Green Tea
Essential oils:
- Lavender
- Rosemary
- Peppermint
Minoxidil
Minoxidil is a universal drug available on the domestic market with a wide spectrum of action.
Minoxidil, a piperidine pyrimidine derivative, an arteriolar vasodilator that activates potassium channels, is catalyzed in NKV by minoxidil sulfotransferase to minoxidil sulfate, an active metabolite that is presumably:
Minoxidil, a piperidine pyrimidine derivative, an arteriolar vasodilator that activates potassium channels, is catalyzed in NKV by minoxidil sulfotransferase to minoxidil sulfate, an active metabolite that is presumably:
- stimulates hair follicles
- increases blood flow
- activates the expression of mRNA of endothelial vascular growth factor in the cells of the dermal papilla, providing an anti-apoptotic effect,
- prolongs anagen,
- leads to a reduction in telogen follicles and an increase in the size of the HF,
- demonstrated an immunoregulatory effect due to an inhibitory effect on T-lymphocytes.
Minoxidil. Clinical researches
• Two studies involving 493 women compared a 5% solution with a 2% solution. Based on hair count data, the 5% solution was marginally more effective than the 2% solution.
• A recent comparative study of Minoxidil 5% Foam and Minoxidil 2% Solution in White patients found similar results.
• Two studies involving 493 women compared a 5% solution with a 2% solution. Based on hair count data, the 5% solution was marginally more effective than the 2% solution.
• A recent comparative study of Minoxidil 5% Foam and Minoxidil 2% Solution in White patients found similar results.
A test is being developed to determine response to minoxidil. The issue of "predictability" of treatment with minoxidil is very relevant, because only 40% of patients can boast of regrowth of hair. At the same time, to evaluate the effectiveness of therapy, one has to wait from 3 to 6 months, all this time taking the medicine “for nothing”. The researchers tried to use the determination of sulfotransferase activity in hair follicles to diagnose the effect of minoxidil earlier.
The essence of the new test is based on the following observations. Minoxidil is converted to minoxidil sulfate in the scalp by the action of the SULT1A1 enzyme. It is assumed that the activity of SULT1A1 in hair follicles correlates with the effectiveness of the drug.
Thus, the use of a sulfotransferase test will help to exclude up to 95% of patients who are resistant to therapy.
The essence of the new test is based on the following observations. Minoxidil is converted to minoxidil sulfate in the scalp by the action of the SULT1A1 enzyme. It is assumed that the activity of SULT1A1 in hair follicles correlates with the effectiveness of the drug.
Thus, the use of a sulfotransferase test will help to exclude up to 95% of patients who are resistant to therapy.
Finasteride and dutasteride
Promising results in the treatment of male AGA are dutasteride, which is used by many dermatologists, despite the fact that the drug is approved for the treatment of this condition only in Korea. Dutasteride has a similar safety profile to finasteride.
Gübelin Harcha et al. in 2014 published the results of a large randomized controlled trial comparing the efficacy of different doses of dutasteride and 1 mg finasteride versus placebo. The authors found that compared with 1 mg of finasteride, the efficacy of taking 0.5 mg of dutasteride was significantly higher. These results further supported the superior efficacy of dutasteride (versus 5 mg finasteride) in increasing hair growth from Olsen's previous randomized controlled trial (Olsen et al. (2006)).
Gübelin Harcha et al. in 2014 published the results of a large randomized controlled trial comparing the efficacy of different doses of dutasteride and 1 mg finasteride versus placebo. The authors found that compared with 1 mg of finasteride, the efficacy of taking 0.5 mg of dutasteride was significantly higher. These results further supported the superior efficacy of dutasteride (versus 5 mg finasteride) in increasing hair growth from Olsen's previous randomized controlled trial (Olsen et al. (2006)).
Topical finasteride
Scientists from Switzerland, taking care of the safety of AGA treatment, have developed a new patented product P-3074, which is a 0.25% finasteride lotion for topical use. In clinical testing, it reduces the concentration of dihydrotestosterone in the scalp by 40% more than oral finasteride. Topical preparations with a combination of minoxidil and finasteride are of interest.
Topical melatonin
The described powerful antioxidant properties of melatonin allow us to consider it as a possible option to counteract oxidative stress associated with involutional hair thinning in AGA and as a preventive measure in graying.
Recently, topical preparations of melatonin have entered the European market as an anti-aging cosmetic product. Topical melatonin has been shown to suppress UV-induced erythema threshold and also increases the proportion of anagen hair in women with AGA.
These data are supported by the results of a double-blind, placebo-controlled pilot study in which topical application of 1 ml of an alcoholic solution of melatonin 0.1% in women with AGA and diffuse alopecia resulted in a significant increase in anagen hair after six months of application compared with placebo.
Recently, topical preparations of melatonin have entered the European market as an anti-aging cosmetic product. Topical melatonin has been shown to suppress UV-induced erythema threshold and also increases the proportion of anagen hair in women with AGA.
These data are supported by the results of a double-blind, placebo-controlled pilot study in which topical application of 1 ml of an alcoholic solution of melatonin 0.1% in women with AGA and diffuse alopecia resulted in a significant increase in anagen hair after six months of application compared with placebo.
Oral antiandrogens
Alternatives to minoxidil include cyproterone acetate, spironolactone, and flutamide.
Corticosteroids
It promotes re-growth in alopecia area by exerting an immunosuppressive effect. Hydrocortisone acetate (25mg/ml) and triamcinolone acetonide (5-10 mg/ml), betamethasone dipropionate 0.05% and fluocinolone acetonide (0.2%) are commonly used.
Adjuvant treatments for AGA and mechanisms of action

Аdjuvant therapy
Currently, the efficacy and safety of minoxidil 2% and 5% twice daily solution for the treatment of AGA is of the highest level of evidence.
The results of the use of pharmacological agents approved for the treatment of other diseases, such as oral antiandrogens, are controversial.
If drug therapy is ineffective, surgical treatment is recommended, although in this case there is a high variability of results.
Several promising methods have recently been proposed.
The results of the use of pharmacological agents approved for the treatment of other diseases, such as oral antiandrogens, are controversial.
If drug therapy is ineffective, surgical treatment is recommended, although in this case there is a high variability of results.
Several promising methods have recently been proposed.
Platelet-rich plasma (PRP)
In the absence of an established optimum concentration level, the currently used PRP preparation method achieves an enrichment of 300-700% (typically over 1,000,000 platelets/µl).
The possible effect of PRP on hair growth has been studied since 2012 in in vitro and in vivo studies in mice.
The actual mechanisms of action on the hair follicle remain controversial: PRP in vitro activates the proliferation of dermal papilla cells and prevents apoptosis, which provokes an increase in the expression level of Akt and Bcl-2. In addition, PRP is involved in the formation of the hair epithelium and the differentiation of stem cells into hair follicle cells. An increase in FGR-7 expression leads to an extension of the anagen phase in the hair growth cycle.
The published results of only a small number of clinical trials of the effectiveness of PRP for hair growth cannot be considered objective. Of the 14 trials included in the systematic review by Gkini et al., only 2 trials in women were evaluated according to the principles of evidence-based medicine.
The possible effect of PRP on hair growth has been studied since 2012 in in vitro and in vivo studies in mice.
The actual mechanisms of action on the hair follicle remain controversial: PRP in vitro activates the proliferation of dermal papilla cells and prevents apoptosis, which provokes an increase in the expression level of Akt and Bcl-2. In addition, PRP is involved in the formation of the hair epithelium and the differentiation of stem cells into hair follicle cells. An increase in FGR-7 expression leads to an extension of the anagen phase in the hair growth cycle.
The published results of only a small number of clinical trials of the effectiveness of PRP for hair growth cannot be considered objective. Of the 14 trials included in the systematic review by Gkini et al., only 2 trials in women were evaluated according to the principles of evidence-based medicine.
Lasers
Lasers and light therapy devices use monochromatic light sources with a wavelength of 600 to 1400 nm in the red/infrared region of the spectrum.
The use of low-intensity laser therapy (LLT), in particular, represents a new approach to the treatment of certain hair diseases, including androgenetic alopecia. Despite the lack of a clear understanding of the mechanism of action, laser therapy stimulates the re-transition of telogen hair follicles into the anagen phase, increases the duration of the anaphase by stimulating epidermal stem cells in the region of the hair follicle bulge, and prevents the premature onset of the catagen phase.
In addition, this type of therapy has been found to regulate the anti-inflammatory and immunological response.
In 2011, the US Food and Drug Administration (FDA) approved the use of the Hairmax Lasercomb R laser comb for the treatment of female AGA, but there are no published results from studies of its effectiveness in women.
Despite numerous studies, more data are needed to develop a standardized treatment procedure, including the optimal wavelength, degree of coherence, and dosimetric parameters.
The safety and possible efficacy of LILT as a treatment option for patients with AGA who are unresponsive or intolerant to standard therapy should be confirmed in further clinical trials.
The use of low-intensity laser therapy (LLT), in particular, represents a new approach to the treatment of certain hair diseases, including androgenetic alopecia. Despite the lack of a clear understanding of the mechanism of action, laser therapy stimulates the re-transition of telogen hair follicles into the anagen phase, increases the duration of the anaphase by stimulating epidermal stem cells in the region of the hair follicle bulge, and prevents the premature onset of the catagen phase.
In addition, this type of therapy has been found to regulate the anti-inflammatory and immunological response.
In 2011, the US Food and Drug Administration (FDA) approved the use of the Hairmax Lasercomb R laser comb for the treatment of female AGA, but there are no published results from studies of its effectiveness in women.
Despite numerous studies, more data are needed to develop a standardized treatment procedure, including the optimal wavelength, degree of coherence, and dosimetric parameters.
The safety and possible efficacy of LILT as a treatment option for patients with AGA who are unresponsive or intolerant to standard therapy should be confirmed in further clinical trials.
Dutasteride in mesotherapy
A recent study examined the efficacy of topical injections of dutasteride in the treatment of female pattern hair loss in 126 patients. A combination of 0.5 mg of dutasteride, 20 mg of biotin, 200 mg of pyridoxine and 500 mg of D-panthenol in a 2 ml solution was injected into the crown area intradermally by mesotherapy. The injections were repeated weekly for 8 weeks, then every 2 weeks for 4 weeks, at the 16th week the last session was performed.
It was found that 18 weeks after the start of treatment (compared to the control group treated with saline), this method had a positive effect on hair growth in women with PVAT. At the 18th week, 62.8% of patients showed photographic improvement, an increase in hair diameter and a decrease in their loss.
It was found that 18 weeks after the start of treatment (compared to the control group treated with saline), this method had a positive effect on hair growth in women with PVAT. At the 18th week, 62.8% of patients showed photographic improvement, an increase in hair diameter and a decrease in their loss.
Prostaglandin analogs
Prostaglandin analogues (PGAs), such as latanoprost and bimatoprost, are topical agents for the treatment of glaucoma and intraocular hypertension. Later it was found that these substances promote the growth and pigmentation of eyelashes. The mechanism of action that promotes hair regrowth is presumably the stimulation of the dermal papilla, leading to the activation of the transition of telogen hair into the anagen phase.
To date, there are no data on the use of APG for the treatment of female AGA.
The use of bimatoprost 0.03% scalp injections once a week for 12 weeks and then every other week for 4 weeks in a 59-year-old female patient with PVAT was unsuccessful.
To date, there are no data on the use of APG for the treatment of female AGA.
The use of bimatoprost 0.03% scalp injections once a week for 12 weeks and then every other week for 4 weeks in a 59-year-old female patient with PVAT was unsuccessful.
Microneedle therapy
Based on the proposed effect on the release of platelet-derived growth factors, stem cell activation, and overexpression of genes associated with hair growth, microneedling therapy has been proposed as a new treatment for AGA.
A randomized trial of 100 men with mild to moderate AGA showed that the combined use of dermaroller and minoxidil 5% lotion in promoting hair growth was significantly superior to that of minoxidil alone.
Despite these promising results, the use of this method in the treatment of AGA needs to be confirmed in clinical trials.
A randomized trial of 100 men with mild to moderate AGA showed that the combined use of dermaroller and minoxidil 5% lotion in promoting hair growth was significantly superior to that of minoxidil alone.
Despite these promising results, the use of this method in the treatment of AGA needs to be confirmed in clinical trials.
Hair transplant
If standard treatments fail to improve hair loss, hair transplantation may be considered as a treatment option. This still effective procedure must be performed by an experienced surgeon, during which individual hair follicles are transplanted from a donor site, but unlike men, in women, due to the diffuse nature of hair loss, the area of \u200b\u200bthe site is very limited.
Among the complications of the operation: temporary hair loss after transplantation, infections, pain and failure of the transplant.
Hair transplantation is performed by various methods, one of such methods is PL-FUT
longitudinal partial follicular unit transplantation.
The new method consists in partial longitudinal extraction of a follicular unit, which can be used as a complete follicular unit to form a fully differentiated hair follicle. The partial follicular unit remaining in the dermis at the donor site can survive and form a hair.
Among the complications of the operation: temporary hair loss after transplantation, infections, pain and failure of the transplant.
Hair transplantation is performed by various methods, one of such methods is PL-FUT
longitudinal partial follicular unit transplantation.
The new method consists in partial longitudinal extraction of a follicular unit, which can be used as a complete follicular unit to form a fully differentiated hair follicle. The partial follicular unit remaining in the dermis at the donor site can survive and form a hair.
Hair transplant - is there an alternative?
Modern areas of research and future methods of therapeutic intervention are:
Accurate identification and effective delivery of the appropriate defense and trigger mechanisms that will stimulate the activation of quiescent stem cells can make the prevention and reversal of hair loss a reality.
- stem cells of follicular origin,
- as well as technologies for cultivating hair follicle tissues.
Accurate identification and effective delivery of the appropriate defense and trigger mechanisms that will stimulate the activation of quiescent stem cells can make the prevention and reversal of hair loss a reality.
Conclusions
AGA can affect men and women, in both similar and completely different patterns.
AGA is characterized by
(1) an increased telogen:anagen ratio, and
(2) hair follicle miniaturization.
Dermal sheath thickening and perifollicular fibrosis are also present in balding regions, and may partly explain the chronic, progressive nature of AGA.
Both genetics and androgens are established as causally linked to AGA. Androgen receptor density, type II 5α-reductase activity, and DHT are elevated in balding scalps.
The DHT “genetic sensitivity” argument for AGA is incomplete, and for several reasons.
(1) Elevated whole-body DHT and polymorphisms in the 5α-reductase gene are not associated with AGA.
(2) Female pattern hair loss has been observed in women who cannot produce androgens.
(3) More recent research now implicates non-androgenic factors like retinoid receptors and PPAR pathways in the onset of hair follicle miniaturization from AGA.
The current model of AGA doesn’t fully explain
(1) what causes DHT to increase in balding regions,
(2) how DHT actually miniaturizes hair follicles,
(3) why DHT is associated with scalp hair loss, but body and facial hair growth, and
(4) why there is a unique pattern and progression to AGA.
Wnt-β catenin signaling, galea interactions, and olfactory receptors are a few new research areas in AGA that might help explain part of the unexplained phenomenon in AGA.
(1) Wnt/β-catenin signaling regulates the stem cell activity in hair follicles needed for follicle regeneration and anagen re-entry. Androgens stimulate DKK-1 secretion by dermal papilla cells which inhibits Wnt/β-catenin signaling and impairs stem cell activity.
(2) Mechanical tension in the galea, which directly corresponds to the patterning in AGA, may play some sort of regulatory role in the inflammation, free radical production, and TGF-β1 expression that seems to mediate scarring and hair follicle miniaturization. However, research here is limited.
(3) Activation of one olfactory receptor by short-chain fatty acids, scalp microflora metabolites, and specific scents may prolong the anagen phase, providing one possible avenue for counteracting AGA progression outside of the traditional anti-androgen treatment approach.
AGA researchers are now turning focus toward non-androgenic factors – PPAR pathways, retinoid receptors, and more – to explain the unexplainable in AGA pathology. If research keeps heading in this direction, newer treatment options will likely better target AGA’s step-processes, resulting in better hair recovery and with a greatly reduced potential for side effects.
AGA is characterized by
(1) an increased telogen:anagen ratio, and
(2) hair follicle miniaturization.
Dermal sheath thickening and perifollicular fibrosis are also present in balding regions, and may partly explain the chronic, progressive nature of AGA.
Both genetics and androgens are established as causally linked to AGA. Androgen receptor density, type II 5α-reductase activity, and DHT are elevated in balding scalps.
The DHT “genetic sensitivity” argument for AGA is incomplete, and for several reasons.
(1) Elevated whole-body DHT and polymorphisms in the 5α-reductase gene are not associated with AGA.
(2) Female pattern hair loss has been observed in women who cannot produce androgens.
(3) More recent research now implicates non-androgenic factors like retinoid receptors and PPAR pathways in the onset of hair follicle miniaturization from AGA.
The current model of AGA doesn’t fully explain
(1) what causes DHT to increase in balding regions,
(2) how DHT actually miniaturizes hair follicles,
(3) why DHT is associated with scalp hair loss, but body and facial hair growth, and
(4) why there is a unique pattern and progression to AGA.
Wnt-β catenin signaling, galea interactions, and olfactory receptors are a few new research areas in AGA that might help explain part of the unexplained phenomenon in AGA.
(1) Wnt/β-catenin signaling regulates the stem cell activity in hair follicles needed for follicle regeneration and anagen re-entry. Androgens stimulate DKK-1 secretion by dermal papilla cells which inhibits Wnt/β-catenin signaling and impairs stem cell activity.
(2) Mechanical tension in the galea, which directly corresponds to the patterning in AGA, may play some sort of regulatory role in the inflammation, free radical production, and TGF-β1 expression that seems to mediate scarring and hair follicle miniaturization. However, research here is limited.
(3) Activation of one olfactory receptor by short-chain fatty acids, scalp microflora metabolites, and specific scents may prolong the anagen phase, providing one possible avenue for counteracting AGA progression outside of the traditional anti-androgen treatment approach.
AGA researchers are now turning focus toward non-androgenic factors – PPAR pathways, retinoid receptors, and more – to explain the unexplainable in AGA pathology. If research keeps heading in this direction, newer treatment options will likely better target AGA’s step-processes, resulting in better hair recovery and with a greatly reduced potential for side effects.